KEYTRUDA® (pembrolizumab) 100 mg/4 mL concentrate solution for infusion is a Prescription Medicine. KEYTRUDA has risks and benefits. The KEYTRUDA Consumer Medicine Information (CMI) is available at www.medsafe.govt.nz or by clicking the 'Consumer Medicine Information' button at the top of this page.
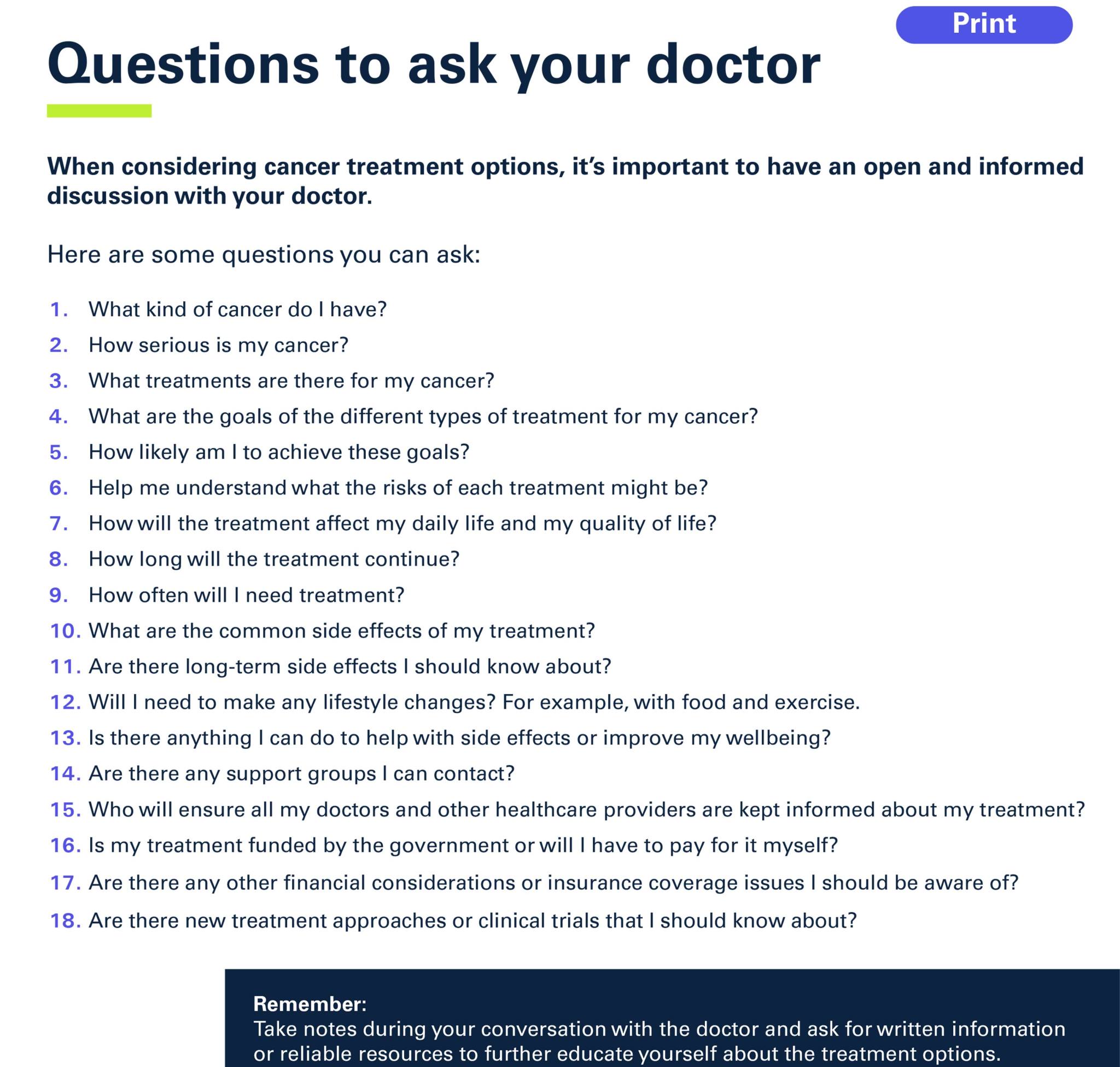
IMPORTANT SAFETY INFORMATION
You should not be given KEYTRUDA if you are allergic to pembrolizumab or to any of the following inactive ingredients: histidine, histidine monohydrochloride monohydrate, sucrose, polysorbate-80, water for injection.
Tell your doctor if you have a disease of your immune system such as Crohn’s disease, ulcerative colitis, or lupus; have had an organ transplant or have had a bone marrow (stem cell) transplant that used donor stem cells (allogeneic); have pneumonia or swelling of your lungs (called pneumonitis); or have liver damage.
If you are pregnant, think you may be pregnant or are planning to have a baby, tell your doctor. KEYTRUDA can cause harm or death to your unborn baby. Effective contraception must be used during treatment with KEYTRUDA and for at least 4 months after the last dose of KEYTRUDA for woman who could become pregnant.
If you are breastfeeding, tell your doctor. Do not breastfeed while taking KEYTRUDA.
Tell your doctor if you are taking any other medicines, including medicines that can be bought without a prescription from a pharmacy, supermarket or health food shop, or other medicines that make the immune system weak. Examples of these may include steroids, such as prednisone.
What are the possible side effects of KEYTRUDA?
KEYTRUDA can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These side effects can sometimes become life-threatening and can lead to death. These side effects may happen anytime during treatment or even after your treatment has ended. You may experience more than one side effect at the same time. Getting medical treatment right away may help keep these problems from becoming more serious.
Call or see your doctor right away if you develop any of the following symptoms:
Lung problems:
Signs and symptoms of lung problems may include shortness of breath, chest pain, or coughing.
Intestinal problems:
Signs and symptoms of problems with your intestines may include diarrhoea or more bowel movements than usual, stools that are black, tarry, sticky, or have blood or mucus, or severe stomach pain or tenderness.
Liver problems:
Signs and symptoms of liver problems may include nausea or vomiting, feeling less hungry, pain on the right side of your stomach, your skin looks yellow, the whites of your eyes look yellow, dark urine or you bleed or bruise more easily than normal.
Hormone gland problems (especially the thyroid, pituitary, adrenal glands):
Signs and symptoms of hormone gland problems may include rapid heartbeat, weight loss or weight gain, increased sweating, hair loss, feeling cold, constipation, your voice gets deeper, muscle aches, dizziness or fainting, or headaches that will not go away or unusual headache.
Kidney problems:
Signs and symptoms of kidney problems may include change in the amount or colour of your urine.
Blood sugar problems:
Signs and symptoms of blood sugar problems may include feeling more hungry or thirsty, needing to urinate more often or weight loss.
Skin problems:
Signs and symptoms of skin problems may include rash, itching, blisters, peeling or skin sores, or ulcers in mouth or in lining of nose, throat, or genital area.
Problems in other organs:
Signs and symptoms of problems in other organs may include muscle pain or weakness; changes in eyesight; stomach area pain with nausea and vomiting (pancreatitis); shortness of breath, irregular heartbeat, feeling tired, or chest pain (myocarditis, pericarditis); confusion, fever, memory problems, or seizures (encephalitis); swollen lymph nodes, rash or tender lumps on skin, cough, or eye pain (sarcoidosis); pain, numbness, tingling, or weakness in the arms or legs, bladder or bowel problems including needing to urinate more frequently, urinary incontinence, difficulty urinating and constipation (myelitis); inflammation of the blood vessels (vasculitis); decreased function of the parathyroid gland, which may include muscle cramps or spasms, fatigue and weakness (hypoparathyroidism); inflammation of the stomach lining, which may include severe stomach pain or tenderness, nausea or vomiting (gastritis); destruction of red blood cells, which may include dark urine, pale or yellow skin/eyes, lightheadedness, feeling tired, rapid heartbeat, or shortness of breath (haemolytic anaemia), or pain in the upper right part of the stomach, swelling of the liver or spleen, fatigue, itching or yellowing of the skin or whites of eyes (sclerosing cholangitis); or decreased ability of the pancreas to make digestive enzymes, which may include diarrhoea with loose and oily stools, weight loss, metabolic bone disease, and vitamin or mineral deficiencies (exocrine pancreatic insufficiency).
Infusion (IV) reactions:
Signs and symptoms of infusion reactions may include shortness of breath, itching or rash, dizziness or fever.
Rejection of a transplanted organ:
People who have had an organ transplant may have an increased risk of organ transplant rejection. Your doctor should tell you what signs and symptoms you should report and monitor you, depending on the type of organ transplant that you have had.
Graft-versus-host-disease (GVHD) in people with bone marrow (stem cell) transplant that uses donor stem cells (allogeneic):
GVHD may occur if you had this transplant in the past. Your doctor will monitor you for the following signs and symptoms: skin rash, liver inflammation, abdominal pain, and diarrhoea.
Common side effects:
Very common side effects of KEYTRUDA include diarrhoea, nausea, itching, rash, joint pain, back pain, feeling tired, cough, patches of discoloured skin, stomach pain, decreased levels of sodium in blood, and low levels of thyroid hormone.
The side effects listed below are additional common side effects that may occur when KEYTRUDA is given together with another treatment, in addition to the very common side effects listed above.
With chemotherapy or chemotherapy + radiotherapy: hair loss, vomiting, decreased number of red blood cells, white blood cells and platelets in the blood, mouth sores, fever, decreased appetite, and swelling of the lining of the digestive system (for example mouth, intestines).
With axitinib: high blood pressure, decreased appetite, blisters or rash on palms of your hands and soles of your feet, increased liver enzyme levels, hoarseness, and constipation.
With lenvatinib: high blood pressure, decreased appetite, vomiting, weight loss, headache, constipation, hoarseness, urinary tract infection, stomach-area (abdominal pain), blisters or rash on the palms of your hands and soles of your feet, protein in your urine, increased liver enzyme levels, and feeling weak.
In children, common side effects of KEYTRUDA include fever, vomiting, headache, stomach pain, decreased number of red blood cells, cough, and constipation.
These are not all of the possible side effects of KEYTRUDA. If you have any side effects, talk to your doctor.
KEYTRUDA may be used to treat certain adults with the following types of advanced cancers:
-
- melanoma
-
- non-small cell lung cancer
-
- urothelial carcinoma
-
classical Hodgkin Lymphoma
-
- head and neck squamous cell carcinoma
-
- renal cell carcinoma
-
- oesophageal carcinoma
-
- cervical cancer
-
- endometrial carcinoma
-
- gastric or gastroesophageal junction adenocarcinoma
-
- triple-negative breast cancer
-
- cutaneous squamous cell carcinoma
-
- a kind of cancer that can occur in any part of the body and is shown by a laboratory test to be microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR)
-
- colon or rectal cancer that is shown by a laboratory test to be MSI-H or dMMR
-
Merkel cell carcinoma
-
biliary tract carcinoma
-
malignant pleural mesothelioma
KEYTRUDA may also be used in certain adults:
-
after surgery to remove melanoma, non-small cell lung cancer, or renal cell carcinoma to help prevent the cancer from coming back
-
before surgery to treat triple-negative breast cancer or non-small cell lung cancer and then continued after surgery to help prevent their cancer from coming back
-
- to treat bladder cancer which has not spread to nearby tissues but is at high-risk of spreading and where bladder removal is not preferred
KEYTRUDA may be used in children with classical Hodgkin lymphoma, Merkel cell carcinoma, malignant pleural mesothelioma, MSI-H or dMMR cancers, or after surgery to remove melanoma. It is not known if KEYTRUDA is safe and effective in children with MSI-H or dMMR cancer of the brain or spinal cord (central nervous system cancers).
KEYTRUDA may be given in combination with other anti-cancer treatments. It is important that you also read the Consumer Medicine Information for these other medicines. If you have any questions about these medicines, please ask your doctor.
Based on the KEYTRUDA Consumer Medicines Information and Data Sheet dated 11th August 2025.
Talk to your doctor about whether KEYTRUDA is a suitable treatment option for you.
KEYTRUDA is funded to treat certain patients with the following cancers: melanoma, non-small cell lung cancer, MSI-H or dMMR colorectal cancer, triple-negative breast cancer, head and neck squamous cell carcinoma, urothelial carcinoma, and cHL. Patients must meet specific criteria to qualify for funding.
If KEYTRUDA is not funded for your particular cancer, you will need to pay the full cost of the medicine and its administration. Talk to your doctor about whether you qualify for funding, the cost of the medicine, and any other fees that may apply.
Merck Sharp & Dohme (New Zealand) Limited, Level 3, 123 Carlton Gore Road, Newmarket, Auckland.
TAPS NP23464 TAPS DA 2514KN NZ-KEY-00885v16 Last updated October 2025
Copyright © 2025 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.